Nuestro equipo científico jefe ha revelado un nuevo mecanismo regulador del "eje célula inmunitaria-intestino-cerebro" durante la aparición de la depresión.
El 19 de noviembre de 2025, el grupo de investigación de los profesores Yao Honghong y Han Bing de la Facultad de Medicina de la Universidad del Sudeste, en colaboración con el equipo del profesor Yuan Yonggui del Hospital Zhongda afiliado a la Universidad del Sudeste, publicó en línea en Nature Communications el título "La migración de células CD8+TSCM al intestino a través del eje PPBP-CXCR2 aumenta" con un IF de 16,6/Q1. El artículo de investigación "Susceptibilidad al estrés del huésped mediante la inhibición del ácido homovanílico derivado del microbioma intestinal" se centra enEstudio sobre la regulación transfronteriza de los subconjuntos de células CD8⁺T y la microbiota intestinal. Intento de resolver el misterio central de la aparición de la depresión.
Este estudio, mediante el análisis de muestras clínicas, la verificación con modelos animales y la combinación de técnicas multiómicas, ha revelado por primera vez de forma exhaustiva una nueva vía para la patogénesis de la depresión: el eje "células T de memoria similares a células madre CD8⁺ (células TSCM CD8⁺) - PPBP-CXCR2 - bacterias intestinales - niveles elevados de ácido vainílico (HVA) - neuroinflamación". Proporciona nuevas dianas y una base teórica para el tratamiento preciso de la depresión.
Contenido principal y resultados
En primer lugar, este estudio analizó sistemáticamente el efecto sinérgico de la inmunidad periférica y la microbiota intestinal en la patogénesis de la depresión mediante una combinación de análisis de muestras clínicas multicohorte y experimentos con animales. El equipo de investigación analizó primero las células inmunitarias de sangre periférica de 115 pacientes con depresión y 115 controles sanos, y descubrió que la proporción de células T en la sangre periférica de pacientes con depresión aumentó significativamente y se correlacionó positivamente con la gravedad de los síntomas depresivos. Además, mediante la tecnología de secuenciación de ARN unicelular (scRNA-seq), las células CD8+ TSCM se identificaron con precisión como la subpoblación inmunitaria clave que impulsa la patología depresiva: el número de estas células aumentó significativamente en pacientes con depresión, y su proporción se relacionó estrechamente con síntomas clínicos como la puntuación en la Escala de Depresión de Hamilton (HAMD-24), la sensación de desesperanza y los trastornos del sueño. Y tiene características transcriptómicas únicas.

Figura 1 Las células CD8+TSCM mejoran la susceptibilidad del huésped al estrés
Posteriormente, para rastrear la trayectoria migratoria in vivo de las células CD8+ TSCM, el equipo de investigación empleó una innovadora técnica de imagenología de marcaje inmunitario de cuerpo completo vDISCO y descubrió inesperadamente que estas células no se infiltraban directamente en el cerebro, sino que migraban direccionalmente al intestino a lo largo del eje PPBP-CXCR2. Estudios del mecanismo han confirmado que la proteína básica plaquetaria (PPBP), altamente expresada en las células CD8+ TSCM, se une a su receptor CXCR2 y constituye una vía molecular clave que media la migración celular al intestino. Este proceso migratorio puede bloquearse significativamente mediante la intervención con el inhibidor de CXCR2 SB265610 o el silenciamiento del gen PPBP.
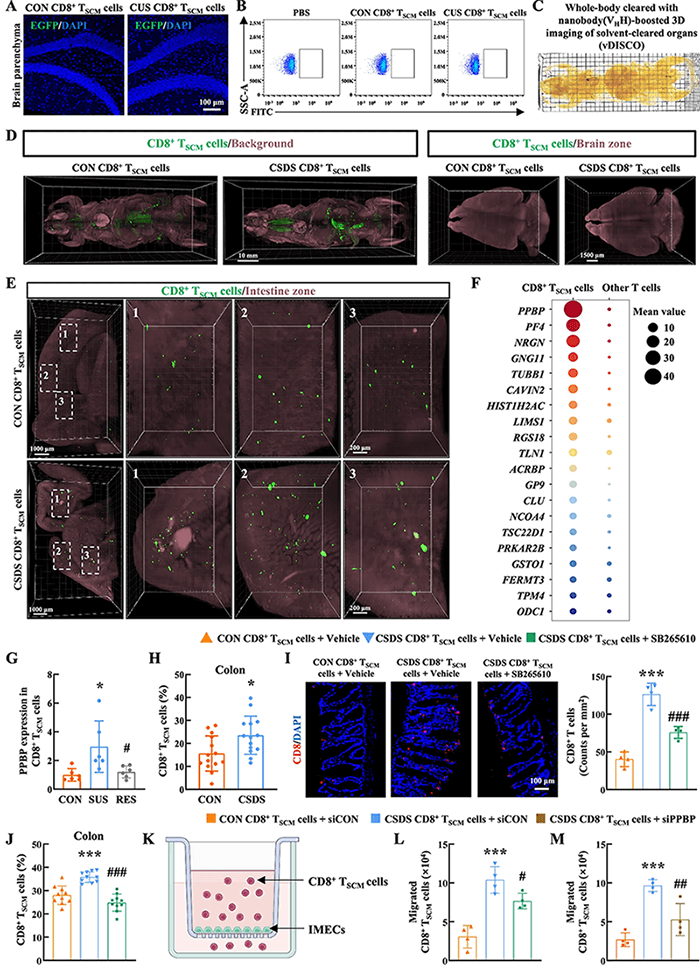
Figura 2 La interacción PPBP-CXCR2 media la migración de células CD8+TSCM al tracto intestinal.
El intestino es el sitio central donde las células CD8+ TSCM ejercen sus efectos patológicos. Investigaciones han demostrado que la migración de células CD8+ TSCM al tracto intestinal puede causar inflamación intestinal, reducir la abundancia de la flora relacionada con la metabolización de la tirosina (especialmente Bifidobacterium skadotropi) y, por lo tanto, conducir a una disminución en la producción de ácido vainílico (AVA), un producto metabólico de la flora intestinal. El AVA, como principal metabolito de la dopamina, se reduce significativamente en la microbiota plasmática e intestinal de pacientes con depresión. La suplementación exógena de AVA puede mejorar eficazmente las conductas de tipo depresivo en ratones con estrés por frustración social crónica (EDCS).

Figura 3 El bloqueo de CXCR2 alivió la reducción de los altos niveles de ácido oxaloico en el cerebro inducidos por células TSCM CD8+ patológicas
Una exploración más profunda del mecanismo indica que una disminución de los niveles de HVA puede inducir neuroinflamación cerebral, lo que provoca una función anormal de los astrocitos y una activación excesiva de la microglía en el hipocampo. Simultáneamente, disminuye la expresión de la proteína asociada a la sinapsis (SYN1) y del factor neurotrófico derivado del cerebro (BDNF), lo que finalmente altera la plasticidad neuronal y desencadena comportamientos depresivos. Los inhibidores de CXCR2 no solo restauran los niveles de HVA, sino que también alivian significativamente la neuroinflamación y mejoran la función sináptica, demostrando un excelente potencial antidepresivo.

Figura 4 El bloqueo de CXCR2 alivió la neuroinflamación y mejoró los síntomas depresivos.
Aspectos destacados de la innovación y su importancia
Este estudio, a través de la cadena completa de investigación clínica, básica y traslacional, ha revelado por primera vez un mecanismo completamente nuevo mediante el cual las células CD8+ TSCM migran a lo largo del eje PPBP-CXCR2 hacia el tracto intestinal, inhibiendo la generación de HVA por la microbiota metabolizadora de tirosina, induciendo así neuroinflamación y depresión. Esto ha roto con la idea tradicional de que la infiltración directa de células inmunitarias en el cerebro provoca depresión. Proporciona una nueva perspectiva cruzada inmunitaria-metabólica para la regulación de la depresión por el eje intestino-cerebro.
La investigación no solo identificó las células CD8+ TSCM como posibles biomarcadores de la depresión, sino que también proporcionó múltiples dianas terapéuticas completamente nuevas. Estos hallazgos proporcionan una base teórica y evidencia experimental importante para el desarrollo de nuevos fármacos antidepresivos, especialmente métodos de intervención para el tratamiento de la depresión resistente.